Our Technology
Seed Amplification Assay:
A Paradigm shift in detection of neurodegenerative diseases
One of the major pathological hallmarks of Parkinson’s disease is the deposition of specific α-synuclein (α-syn) aggregates, commonly referred to as amyloids. These pathogenic amyloids can cross the blood-brain barrier and may circulate in the bloodstream and other body fluids, such as cerebrospinal fluid (CSF).
Recent advances in neurodegeneration research indicate that these amyloid “seeds” can be amplified in vitro to detect the presence of disease, even at ultra-low concentrations. These seeds have a unique property: in the presence of their parent protein, they can trigger a self-propagating cycle of amyloid growth through alternating rounds of incubation and sonication. During incubation, the seeds elongate into filaments; sonication then fragments them into multiple new seeds—amplifying the signal and enabling detection. This principle is well-established in prion diseases and has been adapted for blood- and CSF-based detection using test-tube amplification assays.
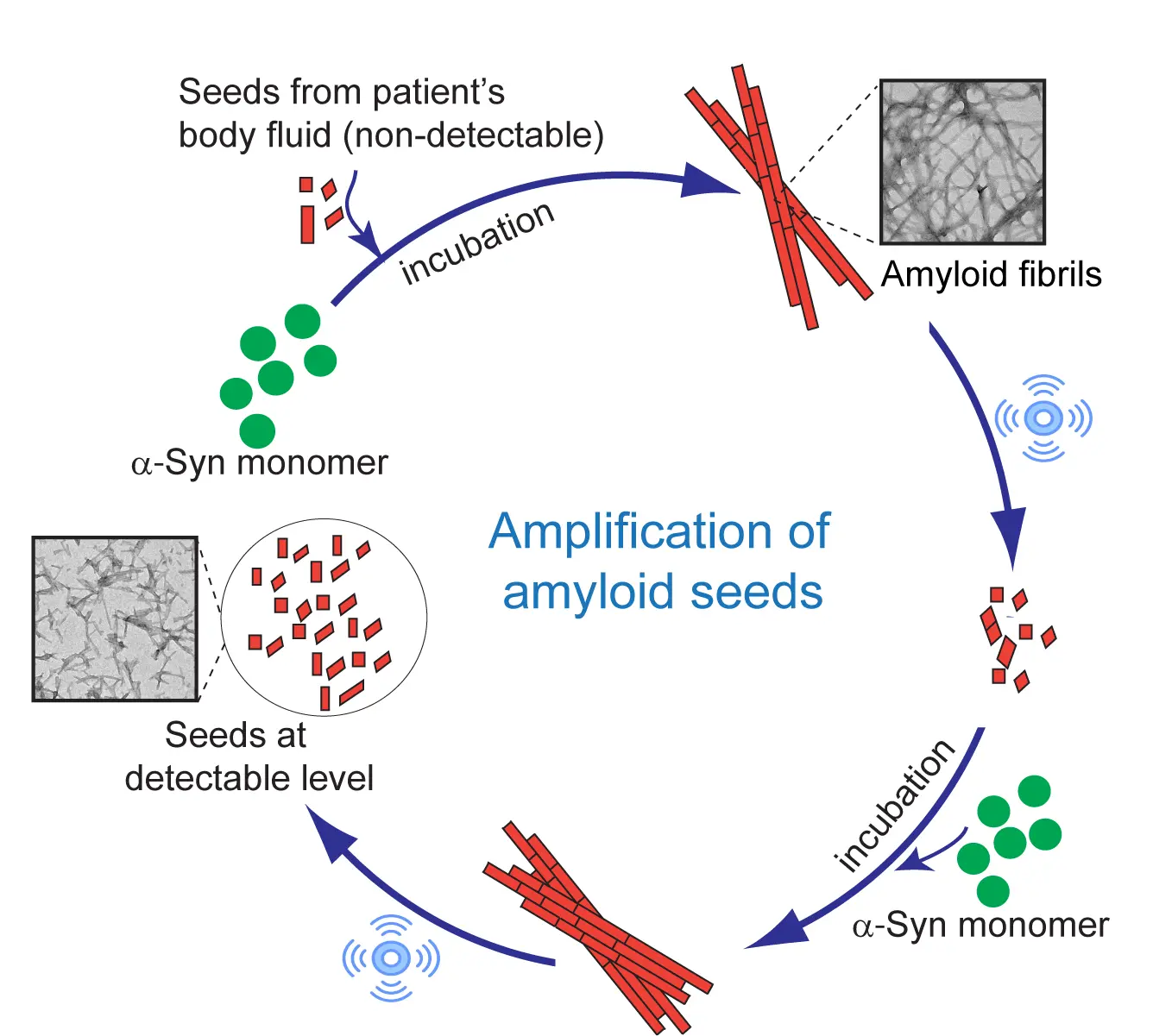
Schematic Representation of Amplification of amyloid seeds through series of sonication-incubation
However, existing seed amplification assays can sometimes produce false positives—particularly when the protein of interest is intrinsically disordered, as is the case with α-synuclein. These proteins may self-aggregate in the test tube, even in the absence of amyloid seeds, limiting the specificity of traditional assays.
UPASANA:
A technique of Unfolded Protein Amyloid Amplification using the Substrate with Altering Nucleation Ability
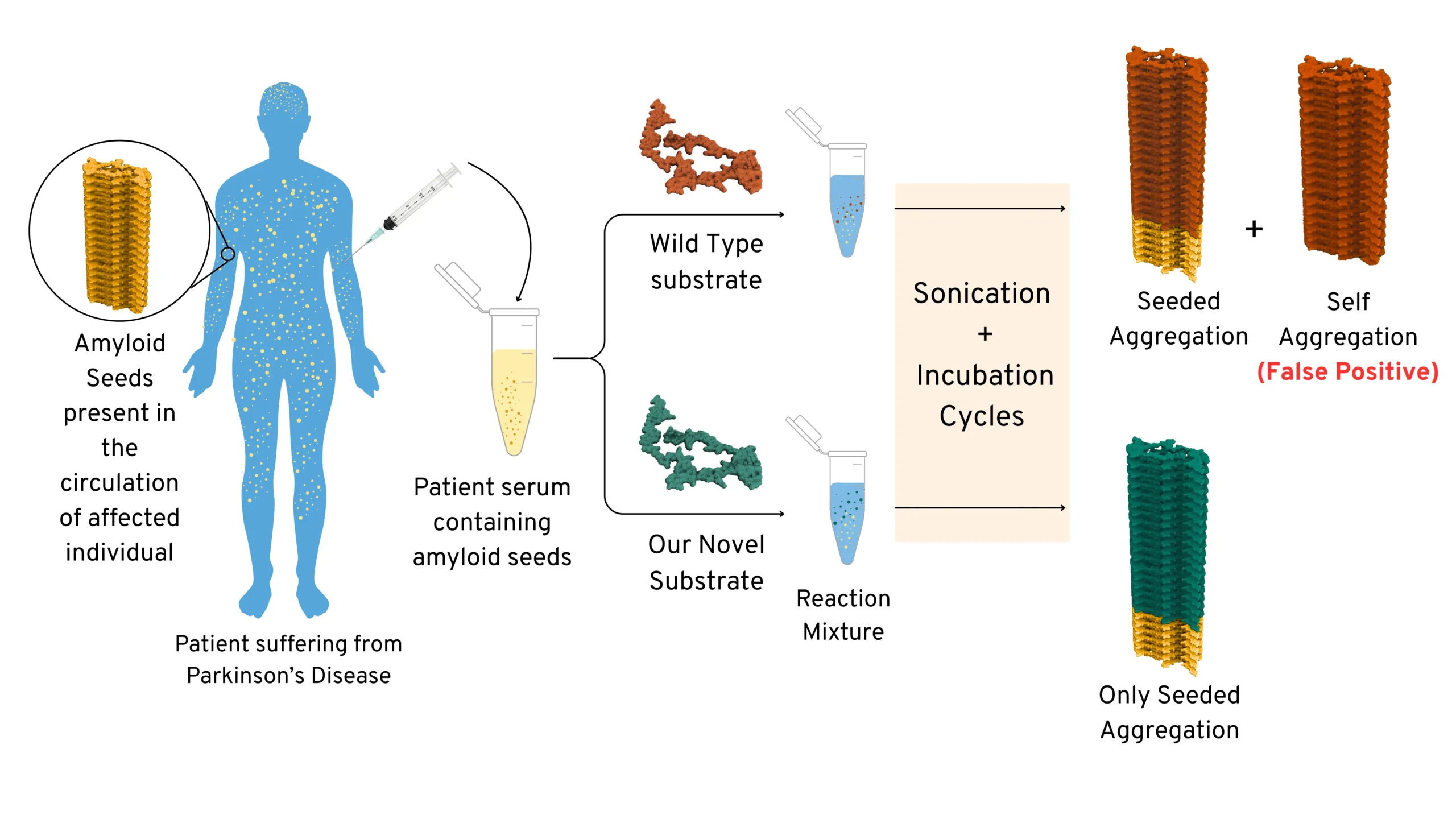
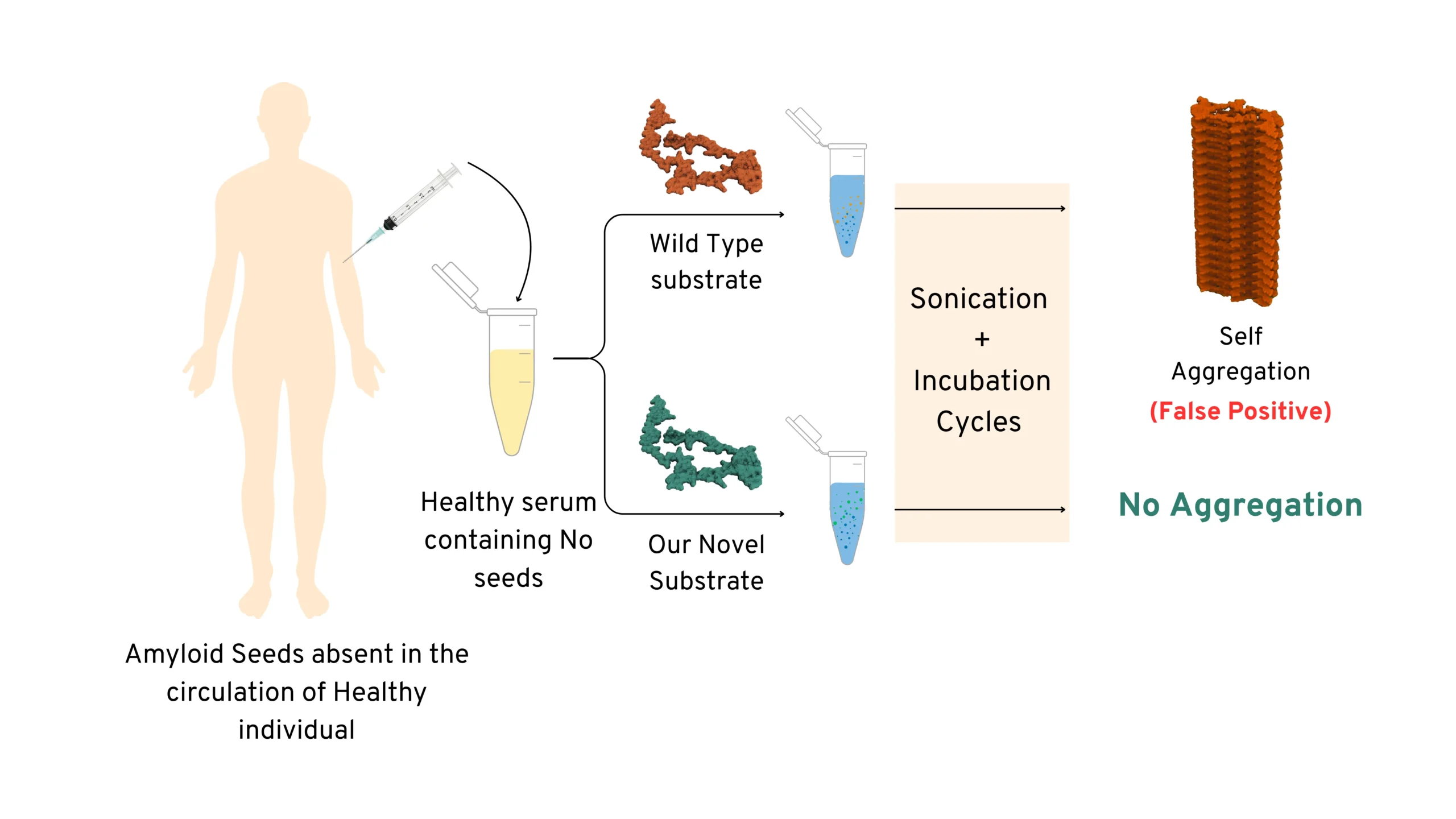
Schematic Representation showing advantage of using our novel substrate (engineered mutant) over using
Wild-Type substrate
To address this, Prof. Samir Maji and his team at IIT Bombay (SCAN Centre and in the Department of Biosciences and Bioengineering) have developed a novel approach. They engineered mutant variants of α-synuclein that behave more like folded proteins, which only participate in amyloid amplification when seeds are present and remain stable otherwise. This technology was termed as UPASANA or “unfolded protein amyloid amplification using the substrate with altering nucleation ability”. The UPASANA mutants show minimal spontaneous aggregation but amplify amyloids efficiently to the detection limit in the presence of pathological seeds (associated with PD)—demonstrating Parkinson’s disease detection.
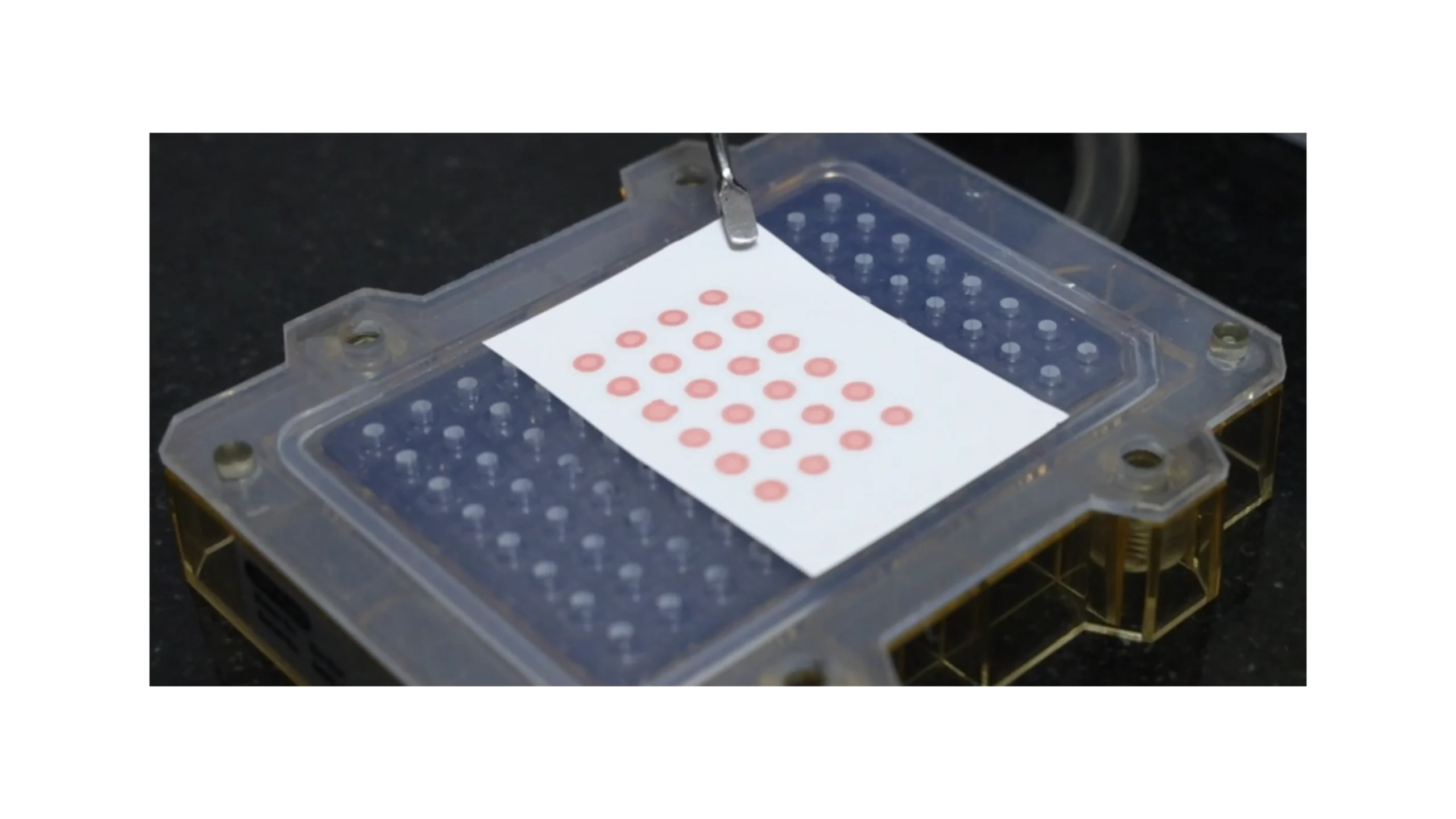
Detection of Misfolded α-Synuclein Aggregates Using High-Throughput Congo Red Dot Blot Assay
Once the misfolded α-synuclein seeds are successfully amplified from the patient’s sample, we use three different technology for detection of the amplified product, one of the methods that we employ is a specialized Congo Red Dot Blot Assay to detect their presence. Congo Red is a dye with a high binding affinity for amyloid aggregates.
In this assay, all sample spots initially show a red signal. However, after an ethanol wash, used to remove non-specific binding, only those samples containing aggregated α-synuclein retain the Congo Red dye. As a result, patient samples with amplified pathological seeds display a distinct red signal, while normal control samples show no residual color.
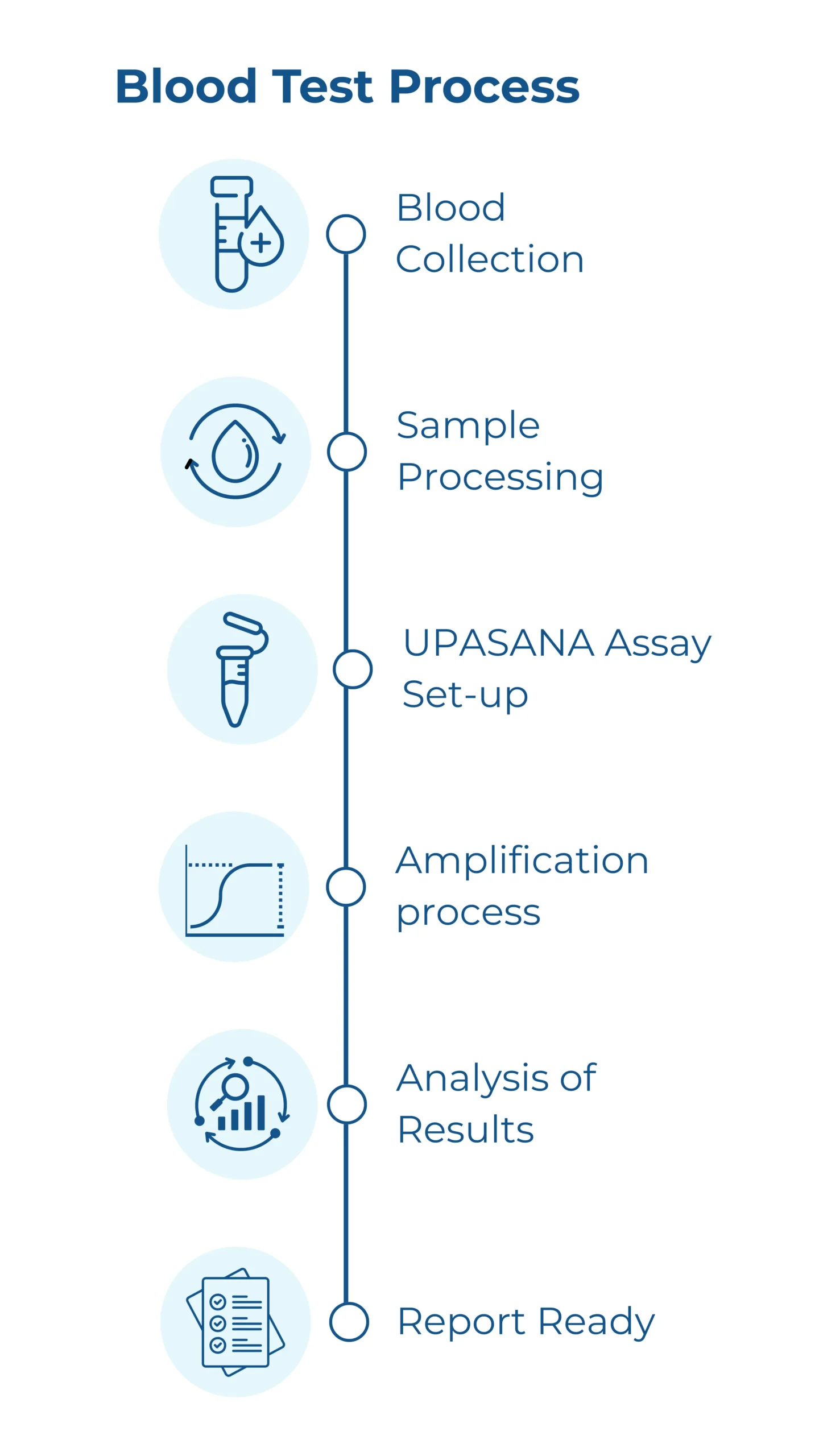
How Our Blood Test Will Work
Blood Collection
A small volume of blood sample is collected from the patient, similar to a routine blood test. This can be done at any partnered hospital after a doctor’s prescription.
Sample Processing
The sample is transported to our central lab under controlled conditions.
UPASANA Assay
The processed sample is introduced to our engineered novel substrate. If Parkinson’s-specific misfolded “seeds” are present, they trigger a chain reaction—amplifying their signal exponentially over time.
Signal Detection & Analysis
Our platform detects aggregation patterns unique to Parkinson’s disease, offering high sensitivity and specificity.
Report Generation
A comprehensive diagnostic report is generated and sent to the prescribing neurologist or hospital within 21 days, aiding in evidence-based decision-making.
Our Progress
- Using this innovation, we have developed a blood-based diagnostic test for Parkinson’s disease.
- Our assay achieved over 90% accuracy in detecting PD in an initial cohort using patient serum samples.
- This test is built on patented technology developed at IIT Bombay, and our findings have been submitted for both Indian and US patents.
The method has now been optimized and is ready for large-scale clinical trials. At AmyScan, we are working to make this a scalable, high-throughput diagnostic platform that can be integrated into clinical workflows.
Detection Technologies Under Development
Pioneering the Future of Neurodiagnostics
Looking ahead, we aim to lead a paradigm shift in the early diagnosis of Parkinson’s disease, enabling detection at a stage where therapeutic interventions may be most effective in slowing disease progression.
In parallel, we are developing tools to differentiate between related disorders—such as Parkinson’s disease, Progressive Supranuclear Palsy (PSP), and other atypical Parkinonisms—to support more accurate diagnosis and treatment pathways.
Related Materials
Understanding Parkinson’s
Understanding how Parkinson’s is Diagnosed in India and what are the available treatment options
About our Technology
Explore how our technology uses α-synuclein’s aggregation behavior to detect tiny amounts of amyloid seeds
About Us
